- DEP® docetaxel phase 1 trial successfully completed with phase 2 to commence immediately
- DEP® docetaxel patients had no reports of neutropenia, a life-threatening toxicity seen in virtually all patients treated with conventional docetaxel formulations (e.g. Taxotere®)
- Encouraging signs of efficacy observed for multiple tumour types with DEP® docetaxel
- Improved pharmacokinetics (PK) of DEP® docetaxel were confirmed in the study showing a longer half‑life, lower peak blood concentration and extended exposure to docetaxel
- Phase 2 trial in lung and prostate cancer to commence immediately with DEP® docetaxel as a monotherapy, and in combination with nintedanib (Vargatef®) for lung cancer
Melbourne, Australia; 22 September 2017: Starpharma (ASX: SPL, OTCQX: SPHRY) today announced that its phase 1 trial for DEP® docetaxel has achieved the key objective of determining a Recommended Phase 2 Dose (RP2D), with no reports of protocol-defined dose limiting toxicities (DLTs). The trial has now been adapted to transition seamlessly into phase 2, with ethics and regulatory approvals already granted, and recruitment and patient screening activities underway for this next phase.
The phase 1 trial enrolled 27 patients with advanced solid cancers, including lung (small cell and non-small cell), prostate, pancreatic, gastro-oesophageal, breast, cervical, renal and brain. Patients received DEP® docetaxel at a range of doses providing the equivalent of 10‑105 mg/m2 docetaxel. Throughout the phase 1 trial, patients were treated with up to six cycles of DEP®docetaxel. Due to the lack of protocol-defined DLTs for DEP® docetaxel, a formal Maximum Tolerated Dose (MTD) was not determined.
The PK characteristics of DEP® docetaxel vary from conventional docetaxel formulations (e.g. Taxotere®), whereby every milligram of docetaxel that is dosed as DEP® docetaxel results in significantly greater exposure to docetaxel than with conventional formulations like Taxotere®. That is, when equivalent[1] doses of DEP® docetaxel and conventional docetaxel formulations are intravenously administered to patients, DEP® docetaxel achieves a much greater exposure to the cancer drug, docetaxel, through its extended half-life.
Despite most trial patients having not responded to or relapsed following previous anti-cancer therapies (i.e., heavily pre-treated prior to enrolment into the trial), encouraging signs of anti-cancer efficacy, including stable disease, were observed in approximately half of the DEP® docetaxel-treated patients for a range of tumour types, including cancers that do not typically respond to docetaxel.
Commenting on the results, Starpharma CEO, Dr Jackie Fairley, said, “It is very exciting to see these phase 1 results for DEP®docetaxel and to move to phase 2. As well as the very promising signs of efficacy observed, what is truly remarkable is that there was not a single report of neutropenia amongst patients dosed with DEP® docetaxel. Neutropenia is a life-threatening side-effect that usually affects more than 90% of Taxotere® patients and often limits the dose that patients can receive.”
“We are also delighted that no hair loss with DEP® docetaxel was reported apart from one patient who experienced a mild case of alopecia. Publications indicate that this side effect is one of the most feared side-effects of cancer treatment. The reduction in these significant side-effects and others such as anaemia, diarrhoea and anaphylaxis means that DEP® docetaxel has the potential to have a positive impact on the quality of life of cancer patients undergoing treatment. This profile also makes DEP® docetaxel an attractive product to combine with other anti-cancer therapies that cause bone marrow toxicity and other toxicities not seen with DEP® docetaxel.”
Phase 1 trial results
Safety and Tolerability
Clear benefits of the DEP® technology were observed in the DEP® docetaxel trial. In particular there were no reports of neutropenia[2], the primary, life-threatening DLT that is reported to occur in virtually all patients receiving 60-100 mg/m2 of conventional docetaxel (Taxotere®). There was also a notable lack of reports of several other problematic adverse events (AEs) observed with docetaxel (e.g. Taxotere®) treatment, including anaphylaxis, fluid retention, diarrhoea and nail disorders. Only one patient treated with DEP® docetaxel experienced anaemia, a very common adverse event associated with docetaxel (Taxotere®) treatment. Furthermore, only one patient treated with DEP® docetaxel experienced a mild case of hair-loss (alopecia), a highly troubling side effect that is reported to occur in three-quarters of patients receiving docetaxel (Taxotere®), with recent reports indicating that such hair-loss can be permanent in some patients.
The most frequently reported AEs observed with DEP® docetaxel included fatigue, rash, peripheral neuropathy, nausea, vomiting, decreased appetite and myalgia. All these AEs are seen with conventional docetaxel (e.g. Taxotere®) and were reported at a comparable or lower rate for DEP® docetaxel than seen with conventional formulations of docetaxel. The majority of these AEs were mild to moderate (grade 1 or 2).
As there were no protocol-defined DLTs in patients across all dose levels, no formal maximum tolerated dose (MTD) was established for DEP® docetaxel in the trial. Instead, the selection of the phase 2 dose (RP2D) of DEP® docetaxel was determined taking account of the overall safety, tolerability, PK and preliminary efficacy results for DEP® docetaxel in the trial. The RP2D has been confirmed as 60 mg/m2 docetaxel equivalents administered intravenously once every 3 weeks.
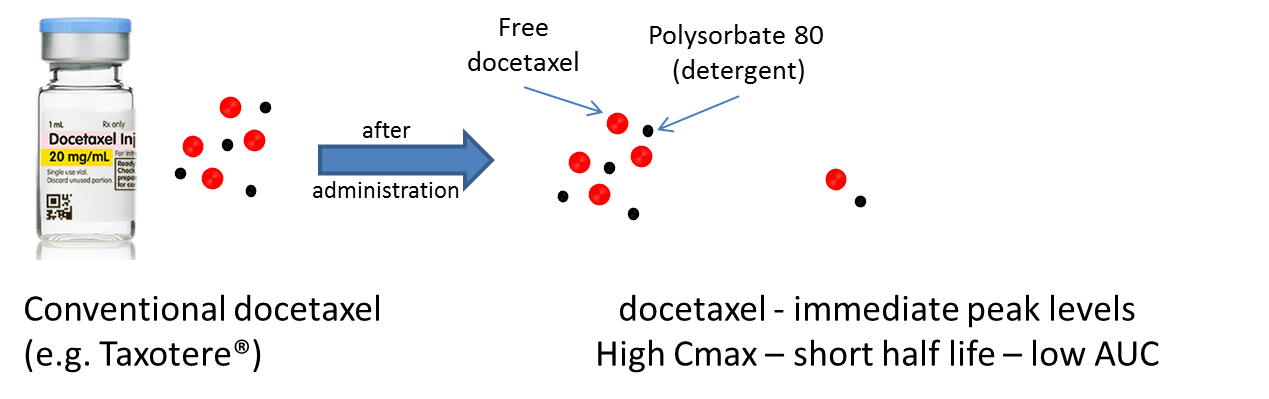
Patients treated with conventional docetaxel formulations (e.g. Taxotere®) are routinely pre-treated with corticosteroids to reduce the risk of life threatening anaphylactic reactions to the excipients (detergents) that are present in conventional docetaxel formulations. Starpharma’s DEP® technology eliminates the need for these detergents as DEP® docetaxel is water soluble, thereby also eliminating the need for steroid pre-treatment in DEP® docetaxel patients, and reducing the risk of anaphylactic reactions.
Efficacy Signals
Whilst this trial was not a formal efficacy study, encouraging signs of efficacy were observed in 13/27 DEP® docetaxel-treated patients, including stable disease in multiple patients with lung, pancreatic and gastro-oesophageal cancers, and in other patients with brain and renal cancers. These responses were observed despite some of these tumour types being typically unresponsive to docetaxel, and most patients having been previously heavily treated with multiple other anticancer therapies, including in some cases taxanes such as docetaxel.
One patient with pancreatic cancer experienced stable disease for more than 20 weeks, and one patient with oesophageal cancer experienced stable disease for more than 18 weeks following treatment with DEP® docetaxel. Substantial decreases in tumour size were also observed for a number of patients.
Pharmacokinetics
The complete set of phase 1 trial PK data support preliminary findings for DEP® docetaxel, and reconfirm that, as intended, the dendrimer acts as a depot of the drug resulting in slow release of the drug (docetaxel) from the dendrimer over time (see diagram below).
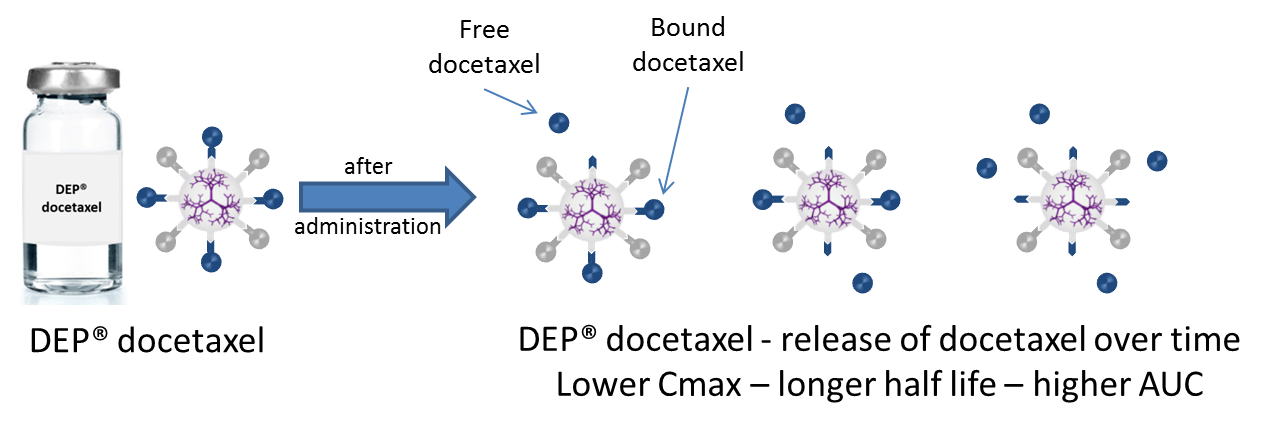
The PK data indicate that the extent of drug exposure, measured as the Area Under the Curve (AUC) for total docetaxel, is substantially higher for DEP® docetaxel than for an equivalent dose of conventional docetaxel.
DEP® docetaxel also results in a significantly lower peak blood plasma level (Cmax) of docetaxel when compared to the peak level for an equivalent dose of docetaxel administered as conventional docetaxel formulations. A reduced Cmax avoids the rapid spike in the concentration of docetaxel in the blood that is thought to be the cause of adverse events such as neutropenia seen with conventional docetaxel treatments.
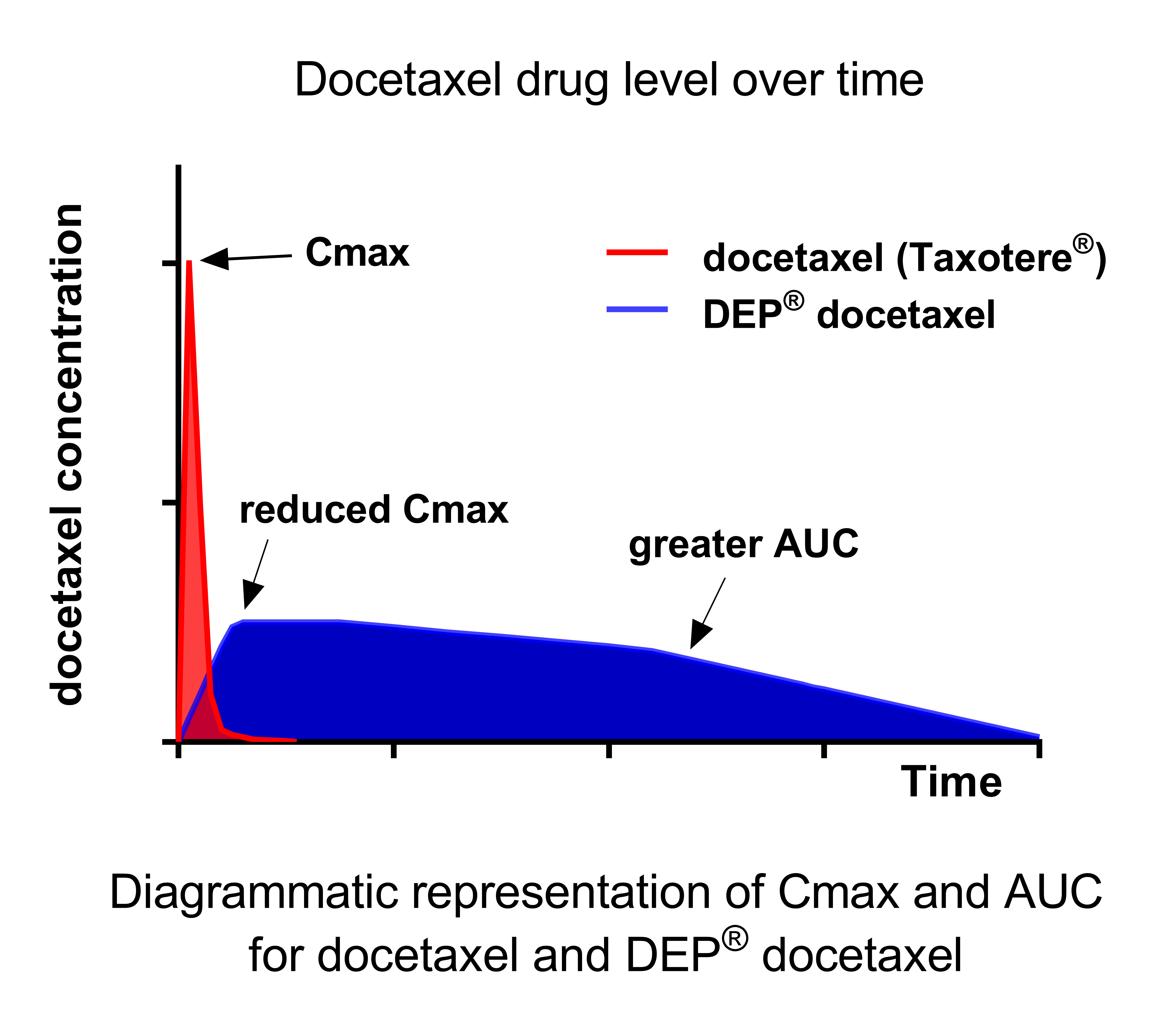
At the RP2D of 60 mg/m2 (see Table 1, below), delivery of docetaxel via the DEP® dendrimer technology significantly increased the plasma half-life (t½) (~9x), reduced the peak plasma level (Cmax) (~50x) and increased docetaxel exposure (AUC) (~650x) when compared with Taxotere®.
Table 1: Pharmacokinetic parameters for DEP® docetaxel compared to Taxotere® (60 mg/m2 docetaxel)¥
| t½ (hours) | t½ (hours)§ | Cmax (ng/mL) | AUC (ng/mL*h) | |
| DEP® docetaxel | 103 | 103 | 50.4† | 2,180,000‡ |
| Taxotere®# | 11.4 | 0.5 | 2,400 | 3,260 |
¥ Comparative data derived from modelling of trial PK results;
§ Plasma half-life for rapid drug elimination phase (applicable to Taxotere® only);
† Cmax, a measure of peak drug level of docetaxel after release from the dendrimer;
‡ AUC, a measure of total drug exposure, derived from plasma drug concentration and time, reflects how much and for how long total docetaxel stays in the body.
# Taxotere® parameters based on published data (Bruno et al, 1996).
Starpharma thanks the DEP® docetaxel study patients, their families and caregivers, and the study staff, investigators and consultants for their participation in this study.
Dr Fairley commented: “The phase 1 results are particularly pleasing given that they are the first human trial results utilising Starpharma’s proprietary DEP® drug delivery platform. The results demonstrate the benefits of our DEP® technology in delivering drugs in a way that modifies conventional pharmacokinetics to reduce certain life-threatening and dose-limiting toxicities. We’re excited to have validated our preclinical findings with DEP® docetaxel and anticipate that the momentum around our internal and partnered DEP® programs will continue to build as we advance this and our other DEP® candidates. These impressive clinical results are not only relevant for DEP® docetaxel itself but also provide important clinical validation that adds further value to Starpharma’s proprietary DEP® platform more broadly.”
“Activities to recruit and screen patients for the phase 2 trial have commenced and we look forward to dosing the first patient in the very near future.”
Phase 2 clinical trial
The phase 2 DEP® docetaxel trial will be conducted at multiple sites, including Guy’s and St Thomas’ Hospital in London, which also participated in the phase 1 study and where recruitment activities for phase 2 have already commenced. Several other sites are currently being opened and recruitment there will begin in the near future.
The phase 2 study is as an open-label, two-stage design, with the objective of establishing anti-tumour activity (efficacy) and safety of DEP® docetaxel at the RP2D of 60 mg/m2. The first stage will enrol approximately 20 patients with lung or prostate cancer, which are key approved indications for docetaxel (Taxotere®). It is intended that the second stage of the trial will enrol a further 20 patients with tumour types selected based on results from the first stage.
In parallel, Starpharma will also investigate the potential benefits of combining DEP® docetaxel with another anticancer agent, nintedanib (Vargatef®), which is approved for treatment of lung cancer in combination with docetaxel (Taxotere®). Up to an additional 12 patients are expected to be enrolled in this arm of the trial.
DEP® docetaxel is Starpharma’s most advanced clinical DEP® candidate and is one of several candidates being developed internally. The next most advanced internal candidate is DEP® cabazitaxel, which is planned to commence a phase 1 clinical trial this year. Starpharma also has a number of partnered DEP® programs, including multiple oncology compounds in development with AstraZeneca and world-leading antibody-drug conjugate companies.
Docetaxel is one of the most widely used cancer drugs for treatment of a wide range of solid tumours including breast, lung and prostate. It is marketed by Sanofi Aventis as Taxotere® and generated peak global sales in excess of US$3 billion.
Download ASX Announcement: DEP® docetaxel positive phase 1 results; phase 2 commences ( pdf file, 363kb)



