Posted: 8 August 2022
Neuren Pharmaceuticals (ASX: NEU) today announced that its Phase 2 clinical trials of NNZ-2591 in children with each of Phelan-McDermid syndrome (PMS) and Pitt Hopkins syndrome (PTHS) are open for enrolment. The first subjects are expected to enter the trials imminently at Rush University Medical Center in Chicago.
The trials are being conducted in the United States under approved Investigational New Drug applications (INDs) with the US Food and Drug Administration. Top-line results from each trial are anticipated for H1 2023.
Neuren CEO Jon Pilcher commented: “We are very excited to be working with the Phelan-McDermid and Pitt Hopkins communities in the United States to undertake these groundbreaking clinical trials. These seriously debilitating conditions have no approved medicines and we are eager to assess the potential impact of NNZ-2591, having observed highly encouraging effects in mouse models of each syndrome.”
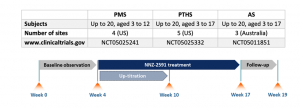
The open label Phase 2 trials will each enrol a single group of up to 20 children to examine safety, tolerability, pharmacokinetics and efficacy over 13 weeks of treatment with NNZ-2591. All subjects will receive NNZ-2591 as an oral liquid dose twice daily, with titration up to the target mg/kg dose during the first 6 weeks of treatment, subject to safety and tolerability. The treatment period is preceded by 4 weeks of observation to thoroughly examine the baseline characteristics prior to treatment against which safety and efficacy will be assessed for each child. A follow-up assessment will be made 2 weeks after end of treatment.
The primary outcome measures for each trial are safety and tolerability, including the incidence, severity and frequency of adverse events, as well as measures of standard pharmacokinetic parameters. Secondary outcome measures include a range of exploratory efficacy measures for each syndrome, completed by clinicians and caregivers.
Neuren also recently commenced a Phase 2 trial of NNZ-2591 in Angelman syndrome (AS). The overall aim of each of these Phase 2 trials is to generate information to inform the design of a subsequent registration trial. In parallel with the Phase 2 trials, Neuren is also executing the foundational work to prepare for Phase 3 development across multiple indications.
About Phelan-McDermid and Pitt Hopkins syndromes
PMS and PTHS are serious neurological disorders affecting both males and females that emerge in early childhood and impact nearly every aspect of life, with no approved medicines.
PMS is caused by a deletion or other change in the 22q13 region of chromosome 22, which includes the SHANK3 gene, or a mutation of the gene. PMS is also known as 22q13 deletion syndrome. The SHANK3 gene codes for the shank3 protein, which supports the structure of synapses between nerve cells in the brain. It is estimated that between 1 in 8,000 and 1 in 15,000 people have PMS. The most common characteristics are intellectual disability, delayed or absent speech, symptoms of autism (approximately 75% are diagnosed with autism spectrum disorder), low muscle tone, motor delays, and epilepsy.
PTHS is a neurodevelopmental condition caused by the loss of one copy or a mutation of the TCF4 gene on chromosome 18. The incidence of PTHS has been estimated at between 1 in 34,000 and 1 in 41,000 people. Characteristics of PTHS are developmental delay with moderate-to-severe intellectual disability and behavioral differences, hyperventilation and/or breath-holding while awake, seizures, gastrointestinal issues, lack of speech, sleep disturbance, stereotypic hand movements and distinctive facial features. Some individuals with PTHS are diagnosed with autism.
About Neuren
Neuren is developing two new drug therapies to treat multiple serious neurological disorders that emerge in early childhood, none of which have any approved medicines.
The lead compound, trofinetide, achieved positive results in a Phase 3 clinical trial for Rett syndrome and has also completed a Phase 2 clinical trial in Fragile X syndrome. Both programs have Fast Track designation from the US Food and Drug Administration (FDA). Neuren has granted an exclusive licence to Acadia Pharmaceuticals Inc. for the development and commercialisation of trofinetide in North America, while retaining all rights outside North America.
Neuren is initiating Phase 2 trials of its second drug candidate, NNZ-2591, for each of Phelan-McDermid syndrome, Angelman syndrome, Pitt Hopkins syndrome and Prader-Willi syndrome.
Recognising the urgent unmet need, all six programs have been granted “orphan drug” designation in the United States. Orphan drug designation provides incentives to encourage development of therapies for rare and serious diseases.
Contact:
Jon Pilcher, CEO
jpilcher@neurenpharma.com
+61 438 422 271