19 February 2020
SYDNEY, AUSTRALIA – Immutep Limited (ASX: IMM; NASDAQ: IMMP) (“Immutep” or “the Company”), a biotechnology company developing novel immunotherapy treatments for cancer and autoimmune diseases, announces interim data from its ongoing Phase II TACTI-002 study. The results are being presented today at the 34th German Cancer Congress in Berlin by Principal Investigator, Dr. Bernhard Doger of START Madrid, Spain. The Company will also present this interim data and provide a further update on its clinical programs in a global webcast, details below.
The data relates to use of the Company’s lead product candidate eftilagimod alpha (“efti” or “IMP321”), a soluble LAG-3 protein, as part of a combination treatment with pembrolizumab. The activation of antigen-presenting cells (APC) and subsequent T cell recruitment with efti may lead to stronger anti-tumour responses than observed with pembrolizumab alone.
Immutep CSO and CMO, Dr Frederic Triebel said: “The results we are seeing from our TACTI-002 trial are highly encouraging, with 47% of first line non-small cell lung cancer patients responding. These results are remarkable given that usually only 20% of patients respond to pembrolizumab monotherapy, if not pre-selected for high PD-L1 expression. Interestingly, patient responses are being seen in all three PD-L1 expression level groups, meaning the combination treatment seems to work even in patients not expected to respond to pembrolizumab monotherapy.
The initial overall response rate of 33% of second line head and neck squamous cell carcinoma patients is also very exciting, albeit from a smaller patient group. It compares well to an expected pembrolizumab monotherapy response rate of 15-18%, especially taking into account that three patients could not yet be assessed.”
Immutep CEO, Marc Voigt stated: “We are very excited by the results from TACTI-002 as pembrolizumab monotherapy is approved only for PD-L1 subgroups in first line NSCLC. Seeing substantial response rates also in low PD-L1 expression groups from the combination therapy is very encouraging, particularly in light of the good safety profile to date for efti.”
Overview of the Trial
TACTI-002 is being conducted in collaboration with Merck & Co., Inc., Kenilworth, NJ, USA (known as “MSD” outside the United States and Canada). It is evaluating the combination of efti with MSD’s KEYTRUDA® (or pembrolizumab, an anti-PD-1 therapy) in up to 109 patients. All patients receive 200 mg of pembrolizumab every three weeks, along with 30 mg of efti every two weeks for the first eight cycles (1 cycle = 3 weeks) and every 3 weeks thereafter (starting cycle 9).
The trial is a Simon’s two-stage, open-label, single-arm study, with patients participating in three Parts:
- Part A – First line Non-Small Cell Lung Cancer (NSCLC), PD-X naive
- Part B – Second line NSCLC, PD-X refractory
- Part C – Second line Head and Neck Squamous Cell Carcinoma (HNSCC), PD-X naive
TACTI-002 is an all comer study in terms of PD-L1 status, a well-known predictive marker for response to pembrolizumab monotherapy especially in NSCLC. PD-L1 expression is typically reported in three groups for NSCLC: < 1%, 1-49% and ≥50% (Tumour Proportion Score or TPS). Patients with a high PD-L1 status are typically more responsive to anti-PD-1 monotherapy such as pembrolizumab, whereas those with low PD-L1 status are overall significantly less responsive. Pembrolizumab monotherapy is registered in the US and the EU for first-line NSCLC patients with a TPS score ≥1% (US) and ≥50% (EU), reflecting 65% and 30% of all first line NSCLC patients, respectively.
Key Findings
Stage 1 Part A (1st line NSCLC):
- Overall Response Rate (ORR) of 47% and no patient with a response had progressive disease thus far
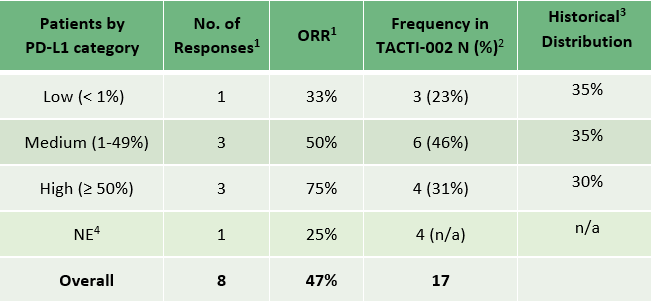
- Distribution of the PD-L1 subgroups is as expected (see table) à ~30% with ≥ 50% PD-L1
- Tumour responses are reported across all three PD-L1 expression level groups (< 1%, 1-49% and ≥50%) for NSCLC. 5 out of the 8 responders had a PD-L1 expression <50%
- Majority (10/17; 59%) of NSCLC patients are still under treatment and median PFS is not yet reached with all patients having passed the 7+ months mark already
- PD-L1 status of stage 1 of Part A (n=17) are detailed below:
Stage 1 Part C (2nd line HNSCC):
- Interim ORR of 33% with 6 out of 18 patients reporting a response according to iRECIST (3 patients are not yet re-staged after initiation of therapy and PD-L1 results are currently being evaluated)
- More detailed data will be provided as patient treatment duration advances.
Safety:
- No new safety signals for this combination therapy reported thus far.
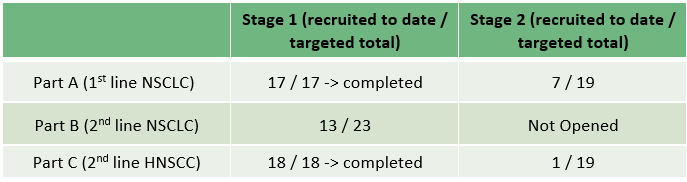
Recruitment Update
Recruitment is ongoing for stage 1 of Part B, along with stage 2 of Parts A and C. The table below summarises the number of patients recruited to date for the TACTI-002 cohorts.
The presentation entitled, ‘Initial results from a Phase II study (TACTI-002) in metastatic non-small cell lung or head and neck carcinoma patients receiving eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab’ will be made available on the Company’s website at https://www.immutep.com/investors-media/presentations.html.
Webcast Details
The Company will also present this interim TACTI-002 data and provide a further update on its clinical programs in a global webcast. The details for the webcast are as follows:
Date & Time: Wednesday, February 26, 2020, 8:00 am Australian Eastern Daylight Time /
Tuesday, February 25, 2020, 4:00 pm US Eastern Daylight Time
Register: Interested parties can register via a link to the webcast on the Company’s website or via the following link: https://fnn.webex.com/fnn/onstage/g.php?MTID=e11208a0ff7fcccaca4d5e4b8a14e988f
Questions: Investors are invited to submit questions in advance via immutep@citadelmagnus.com.
A replay of the webcast will also be available at www.immutep.com from the day after the event.
About the TACT-002 trial
TACTI-002 (Two ACTive Immunotherapies) is being conducted in collaboration with Merck & Co., Inc., Kenilworth, NJ, USA (known as “MSD” outside the United States and Canada). The study is evaluating the combination of efti with MSD’s KEYTRUDA® (or pembrolizumab, an anti-PD-1 therapy) in up to 109 patients with second line head and neck squamous cell carcinoma or non-small cell lung cancer in first and second line. The trial is a Phase II, Simon’s two-stage, non-comparative, open-label, single-arm, multicentre clinical study that is taking place in up to 13 study centres across the U.S., Europe and Australia.
About Immutep
Immutep is a globally active biotechnology company that is a leader in the development of LAG-3 related immunotherapeutic products for the treatment of cancer and autoimmune disease. Immutep is dedicated to leveraging its technology and expertise to bring innovative treatment options to market for patients and to maximize value to shareholders. Immutep is listed on the Australian Securities Exchange (IMM), and on the NASDAQ (IMMP) in the United States.
Immutep’s current lead product candidate is eftilagimod alpha (“efti” or “IMP321”), a soluble LAG-3 protein (LAG-3Ig) based on the LAG-3 immune control mechanism. This mechanism plays a vital role in the regulation of the T cell immune response. Efti is currently in a Phase IIb clinical trial as a chemoimmunotherapy for metastatic breast cancer termed AIPAC; a Phase II clinical trial being conducted in collaboration with Merck & Co., Inc., Kenilworth, NJ, USA (known as “MSD” outside the United States and Canada) referred to as TACTI-002 (Two ACTive Immunotherapies) to evaluate a combination of efti with KEYTRUDA® (or pembrolizumab, an anti-PD-1 therapy) in several different solid tumours (clinicaltrials.gov identifier NCT03625323); a Phase I clinical trial being conducted in collaboration with Merck KGaA, Darmstadt, Germany and Pfizer Inc. referred to as INSIGHT-004 to evaluate a combination of efti with avelumab (clinical trials.gov identifier NCT03252938); and a Phase I combination therapy trial in metastatic melanoma termed TACTI-mel (clinicaltrials.gov identifier NCT02676869).
Additional LAG-3 products, including antibodies, for immune response modulation in autoimmunity and cancer are being developed by Immutep’s large pharmaceutical partners. Immutep is also developing an agonist of LAG-3 (IMP761) for autoimmune disease.
Further information can be found on the Company’s website www.immutep.com or by contacting:
Australian Investors/Media:
Catherine Strong, Citadel-MAGNUS
+61 (0)406 759 268; cstrong@citadelmagnus.com
U.S. Media:
Garth Russell, LifeSci Advisors
+1 (646) 876-3613; garth@lifesciadvisors.com
This announcement was authorised for release by the board of Immutep Limited.
Click here to read the full release: Immutep Reports Positive TACTI-002 Data



