
Posted: 26 April 2023
Starpharma today announces that it has developed a HER2-targeted DEP® SN-38 antibody-drug conjugate (ADC), which has shown significant anti-tumour activity in a HER2+ human ovarian cancer xenograft model, outperforming the approved ADC product, Enhertu®[2].
ADCs represent an innovative and growing area of cancer treatment, with encouraging clinical advances and product approvals in recent years. The global ADC market grew from USD ~$5.8 billion in 2021 to USD ~$8.0 billion in 2022 and is projected to reach USD ~$22.9 billion in 2030[3]. HER2 is an important target for cancer treatments, with ADCs targeting this receptor showing significant promise. Currently marketed HER2-targeted ADCs include Kadcyla® (Genentech and Roche) and Enhertu® (AstraZeneca and Daiichi-Sankyo), which is now approved for use in patients with HER2+ or HER2-low metastatic breast cancer, HER2+ metastatic gastric or gastroesophageal junction cancer, and HER2-mutant metastatic non-small-cell lung cancer[4].
Starpharma’s HER2-targeted DEP® SN-38 ADC comprises a HER2-directed antibody, trastuzumab, linked to DEP® dendrimers loaded with the topoisomerase I inhibitor, SN-38, which is the active metabolite of irinotecan. Starpharma’s HER2-targeted DEP® SN-38 ADC has been designed with a higher drug-to-antibody ratio (DAR), or drug loading, than currently marketed ADCs.
The anti-cancer activity of Starpharma’s HER2-targeted DEP® SN-38 ADC was demonstrated in an established HER2+ human cancer xenograft model, utilising the SKOV-3 ovarian cancer cell line that overexpresses HER2. The HER2-targeted DEP® SN-38 ADC and Enhertu®, dosed intravenously (IV) on study days 1, 8 and 15, significantly inhibited tumour growth compared with saline control. The anti-tumour effect of the HER2-targeted DEP® SN-38 ADC was statistically significantly greater than the anti-tumour effect of Enhertu® over the duration of the study (p<0.0001). All animals in the HER2-targeted DEP® SN-38 ADC group survived to the end-of-study and survival was statistically significantly greater for the HER2-targeted DEP® SN-38 ADC-treated animals compared with Enhertu® and the saline control group (p<0.0002).
Starpharma’s HER2-targeted DEP® SN-38 ADC, containing SN-38, achieved superior tumour growth inhibition and survival compared with Enhertu®, despite the active drug in Enhertu®, DXd, being approximately 10 times more potent against topoisomerase I than SN-38[5].
Key advantages of Starpharma’s DEP® platform for ADCs include its ability to achieve higher DAR, and therefore higher drug payload, than conventional ADCs (see Table 1, below); its greater flexibility in terms of linker strategies to precisely control drug release profiles; and its ability to widen the therapeutic index of toxic drug payloads. In addition, Starpharma’s DEP® platform provides unique flexibility in terms of compatible targeting agents, including biologics (whole antibodies and fragments), small molecules, peptides and other approaches.
Table 1. HER2 ADCs Drug-to-Antibody Ratios (DAR), Drug Payload and Payload Mechanism of Action
| HER2 ADC | Approximate Drug-to-Antibody Ratio (DAR) | Drug Payload | Payload Mechanism of Action |
| Kadcyla®
(Genentech/Roche) |
3.5 | DM-1 (emtansine) | Microtubule
inhibitor |
| Enhertu®
(AstraZeneca/Daiichi-Sankyo) |
8 | DXd (exatecan derivative) | Topoisomerase I inhibitor |
| HER2-targeted DEP®
SN-38 ADC (Starpharma) |
13 | SN-38 | Topoisomerase I inhibitor |
Starpharma CEO, Dr Jackie Fairley, said: “Antibody-drug conjugates are one of the fastest growing classes of anti-cancer drugs, with significant progress being made in recent years, including the recent approval and clinical success of Enhertu®, which has shown significant promise in helping patients with HER2+ cancers.
“Starpharma is pleased to report this new data for its internally developed HER2-targeted DEP® SN-38 ADC demonstrating significant anti-tumour activity and survival, compared to Enhertu®, in a HER2+ cancer model.
“Starpharma’s DEP® technology delivers a number of advantages in the design of innovative ADCs, including the ability to load more drug payload molecules per construct and having greater flexibility in linker strategies. In addition to our internal activities in this area, Starpharma is delighted to be working in partnership with a number of companies, including MSD, to develop dendrimer-based ADCs using Starpharma’s DEP® technology.”
Study Methods
Starpharma evaluated the anti-cancer activity of HER2-targeted DEP® SN-38 ADC compared to Enhertu® in an established HER2+ human cancer xenograft model. The study was conducted at the Monash Institute of Pharmaceutical Sciences (MIPS).
This murine xenograft study used the HER2+ SKOV-3 ovarian cancer cell line, in NOD-SCID-Gamma (NSG) immunodeficient mice. The SKOV-3 cell line was chosen because it naturally overexpresses HER2 and is not dependent on estrogen for growth and survival, meaning that it is a robust model for evaluation of HER2-targeting drugs. Tumour cells were inoculated subcutaneously and the resultant tumours were measured 2-3 times weekly using electronic calipers. Tumour volume (mm3) was calculated at each timepoint.
Following tumour establishment, groups of mice (n = 7-8 per group) were dosed IV on days 1, 8, and 15 as follows:
- Vehicle (saline)
- Control dendrimer-SN-38[6] – 3.28 mg/kg (SN-38 equivalent)
- Enhertu® – 5 mg/kg (total construct dose)[7]
- HER2-targeted DEP® SN-38 ADC – 3.28 mg/kg (SN-38 equivalent)
Study Results
Effect of HER2-targeted DEP® SN-38 ADC vs. Enhertu® on Tumour Volume Over Time
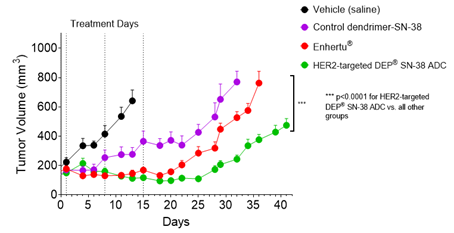
Figure 1. HER2-targeted DEP® SN-38 ADC achieved statistically significantly enhanced tumour growth inhibition compared with Enhertu® or the control dendrimer that lacked the HER2-targeting antibody (p<0.0001 for both comparisons). Points and error bars show means ± standard error of the mean (SEM). Statistical analyses used the Mixed Effects Model (GraphPad Prism v9.4.1).
Kaplan-Meier Survival Curve
Figure 2. Analysis of survival curves showed significantly enhanced probability of survival of the HER2-targeted DEP® SN-38 ADC group versus all other groups (p<0.0002). Probability of survival in the HER2-targeted DEP® SN-38 ADC group was 100% (at end-of-study, 45 days). Median survival, or the time at which probability of survival dropped to 50%, was 40 days for Enhertu®, 28 days for the control dendrimer, and 13 days for the vehicle control. Survival analyses used tumour volume (≥ 1000 mm3), tumour-site ulceration, or end-of-study as endpoints.
Survival analyses used the logrank (Mantel-Cox) test (GraphPad Prism v9.4.1).
All treatments were well tolerated, as measured by change in body weight over time.



