
Posted: 24 July 2023
Starpharma (ASX: SPL, OTCQX: SPHRY) today announces that DEP® HER2-zirconium, its HER2-targeted radiodiagnostic candidate, has demonstrated a favourable biodistribution profile, with excellent imaging contrast between tumour and normal tissues, as well as rapid uptake and high levels of tumour accumulation in a HER+ breast cancer model. These are important features for a radiodiagnostic product for accurate diagnosis and monitoring of tumour lesions using PET-CT imaging. The study details and results are reported below.
Starpharma’s DEP® HER2-zirconium is a radiodiagnostic product that belongs to the rapidly growing “radiotheranostic” category – which includes both radiodiagnostic and radiotherapeutic products. DEP® HER2-zirconium is designed to specifically diagnose, stage, and monitor HER2+ cancers with greater sensitivity, meaning that patients suffering from these cancers could be diagnosed earlier, more accurately, and monitored more closely during cancer treatment.
The study results are very encouraging as they confirm the optimised binding properties of DEP® HER2-zirconium for targeted delivery and preferential uptake by cancer cells, and support a precision medicine approach for cancer patients. The combined effect of the novel pharmacological properties of DEP® HER2-zirconium gives it an advantage in the promotion of selective tumour cell entry and support its targeted delivery mechanism to tumour cells, leaving normal cells relatively untouched.
Commenting on the significance of these findings, Clinical Pharmacology and Oncology specialist, Dr Paul Wabnitz, (MD, FRACP)3, said:
“Translated clinically, this technology has the potential to detect cancer cells at very low levels and better guide therapeutic decisions at earlier stages and at levels that were previously undetectable by current radiological methods. This has several advantages, including dose optimisation and better identifying the minimal dose level for an efficacious response, thereby minimising toxicity and promoting the quality of life and care of cancer patients undergoing therapy.”
Dr Jackie Fairley, CEO of Starpharma, commented:
“Radiotheranostics and HER2 therapeutics are both rapidly growing categories with a number of highly successful product launches in recent years. The application of Starpharma’s DEP® technology in the radiotheranostic area presents a substantial opportunity to expand the commercial opportunity for DEP®. The DEP® platform offers greater flexibility which allows a wide range of radioisotopes to be utilised.”
HER2 and radiotheranostics market
The global radiotheranostics market was US$1.8 billion in 2022 and is expected to grow by over 10% annually to US$4.2 billion by 2030. There are already several successful marketed radiodiagnostics, such as Pylarify® and Illuccix®, which detect prostate cancer through PET-CT scans, with others in development.
HER2-based antibody products had total sales of ~US$11 billion in 2022, which included Herceptin® (or trastuzumab), marketed by Roche/Genentech and the highly successful AstraZeneca product, Enhertu®.
DEP® HER2-zirconium study details and results
DEP® HER2-zirconium utilises a novel single-domain antibody (sdAb), or nanobody, which is smaller than a full mAb, to target and bind to HER2+ tumour cells. The study assessed the biodistribution and imaging (diagnostic) performance of DEP® HER2-zirconium compared with a HER2-targeted full antibody (trastuzumab/Herceptin®), labelled with zirconium-89. Zirconium-89 is a radioisotope used for PET-CT imaging in cancer patients.
A HER2+ human breast cancer cell line (BT474) was grown as a tumour subcutaneously in mice. Biodistribution was measured by in vivo PET-CT imaging and quantitative ex vivo gamma counting at time points ranging from 4 hours to 120 hours after dosing with either DEP® HER2-zirconium or trastuzumab-zirconium, with a target radioactivity of 3 MBq (megabecquerel) per dose.
In this study, DEP® HER2-zirconium:
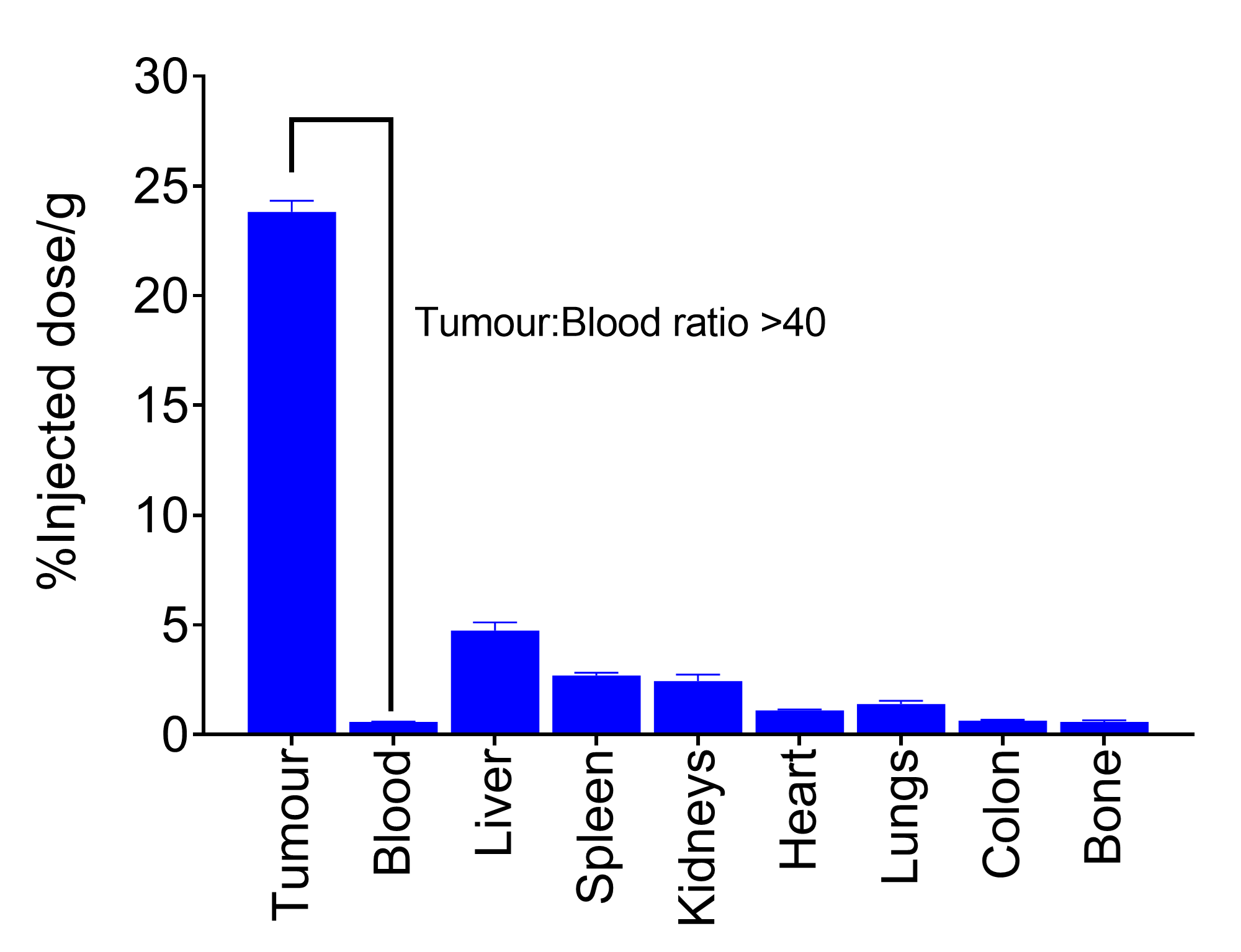
- Showed significant accumulation in tumour (>25% of the injected dose per gram [ID/g] of tumour);
- Was cleared more rapidly from the blood, and highly perfused organs such as heart and lung, than HER2 mAb trastuzumab-zirconium (see Figure 1);
- Demonstrated a significantly higher tumour-to-blood ratio than trastuzumab-zirconium; and
- Resulted in high tumour-to-organ ratios, delivering excellent specificity in imaging the HER2+ breast cancer model (see Figure 2).
Figure 1. Injected dose in blood over time, demonstrating more rapid clearance of DEP® HER2-zirconium versus trastuzumab-zirconium.
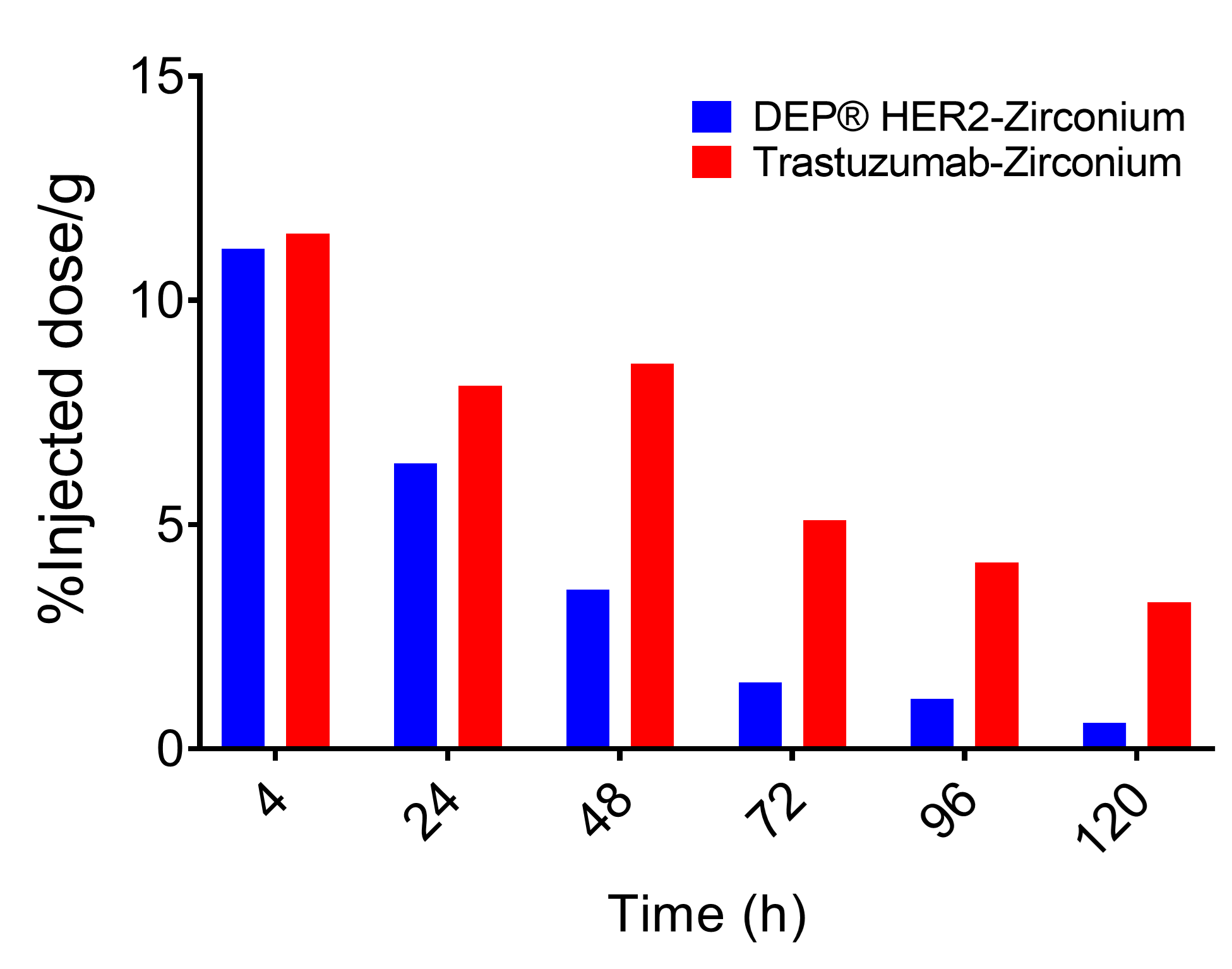
Figure 2. Tumour and normal tissue levels of DEP® HER2-zirconium at 120 hours.
In summary, the data reported here for DEP® HER2-zirconium highlight its promise in radiotheranostics, due to its efficiency of tumour delivery and favourable PK and biodistribution characteristics. The study results demonstrate positive characteristics that are relevant to both radiodiagnostic and radiotherapeutic DEP®-based products.
Starpharma’s DEP® radiotheranostics programs
In addition to the imaging/diagnostic results reported above, the study results also highlight the potential benefits of DEP® technology in radiotherapeutic, or treatment, applications.
Radiotherapeutics seek to deliver radiation directly to cancer cells as a treatment while minimising adverse effects on other organs in the body. Radiotherapeutics utilising mAbs are often associated with dose-limiting haematological toxicities, such as neutropenia and thrombocytopenia, due to the slow clearance of mAbs from the body.
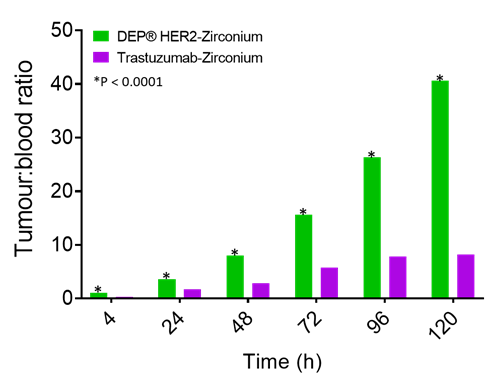
In this study, DEP® HER2-zirconium demonstrated highly desirable “fast-in”/”fast-out” kinetics, meaning it accumulates rapidly in the tumour and is cleared quickly from the bloodstream. DEP® HER2-zirconium achieved 40 times more drug in tumour versus blood, while at the same time point, trastuzumab-zirconium was only 8 times more in the tumour than in the blood (Figure 3).
Figure 3: Tumour-to-blood ratio over time for DEP® HER2-zirconium is significantly larger than trastuzumab-zirconium.
Furthermore, rapid clearance of DEP® HER2-zirconium from the blood suggests that the corresponding DEP® HER2-lutetium radiotherapeutic would be expected to have reduced haematological toxicity compared with mAb-based radiotherapeutics.
For therapeutic applications, Starpharma is developing DEP® HER2-lutetium, which has demonstrated a more favourable PK and tissue biodistribution than a full mAb. This profile has the potential to reduce specific toxicities associated with mAb-based radiotherapeutics. Starpharma has previously reported results for DEP® HER2-lutetium demonstrating enhanced delivery of radioisotopes to solid tumours in pre-clinical studies. These prior data for the DEP® radiotheranostics also demonstrated durable anti-tumour responses, enhanced survival, and high levels of tumour uptake, including in a glioma (brain cancer) model. These two products, DEP® HER2-lutetium and DEP® HER2-zirconium, constitute a HER2 “theranostic pair”.
View or download the ASX Announcement here.



