
Posted: 20 February 2023
Training for those Experienced in GMP, up to Senior Level Managers
Current GMP requirements are increasingly demanding and complex and getting it right first time has significant impacts on quality, regulatory compliance and business efficiency. Modern managers need to understand and work across different disciplines as part of a cohesive team. The six short courses are based on consultation with industry and our consulting experience where we often see knowledge gaps and sub-optimal application of these disciplines. In particular, the application of risk management has significant leverage over the other five topics and will be a common theme throughout the series.

Advanced GMP Program Outline
The six courses are complementary and designed to super charge attendees for a more in depth understanding of critical areas that underpin GMP compliance in practice.
Delivered in a virtual, online environment, ideal for busy professionals. Theoretical concepts will be translated to the real world environment and be supported with Guest lectures, self directed learning, Q&A facilitated sessions and case work. A minimum of 12 months working in a GMP role or the GMP Essentials Program are pre-requisites for the course.
There is an online Welcome and Information Session, then each online Short Course will entail:
- Two to three hours of theory, practical application and guest lectures (self paced learning)
- One hour of facilitated Q&A Session with a CBE expert
- Case study work
- An online Assessment
Each Short Course will have an assessment and at completion of the Advanced GMP Uplift Program participants will receive a Certificate. For REDI eligible participants, the majority of the program costs are covered by MTPConnect REDI Initiative, however there is a small co-payment of $200 per participant.
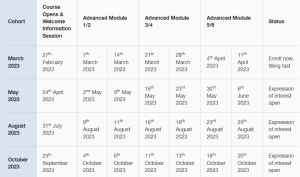
Advanced GMP Program Schedule
The schedule for the 2023 Advanced GMP Program is now available, places per cohort are limited, so register your interest today to secure a place or email gmpuplift@cbe-ap.com.au with any queries.
Find out more and click here to register for a place in the GMP Uplift Program.



