 Posted: 7 July 2023
Posted: 7 July 2023
ASX Announcement
Key points:
- New data favourably links AD-214 preclinical animal studies and Phase I human clinical trial results to provide important dose frequency insights
- AD-214 is designed to treat fibrotic diseases such as Idiopathic Pulmonary Fibrosis (IPF) by inhibiting the effect of the chemokine receptor CXCR4
- The new data links CXCR4 receptor occupancy with CXCR4 inhibition to provide direct evidence suggesting that administration of AD-214 every two weeks could be clinically feasible
- Findings de-risk and improve confidence in AD-214 Phase II clinical study dosing regimens, addressing a key partner question.
MELBOURNE Australia, 7 July 2023
AdAlta Limited (ASX:1AD), the clinical stage drug discovery company developing novel therapeutic products from its i-body platform, is pleased to announce new data supporting the potential efficacy of AD-214 in human patients with Idiopathic Pulmonary Fibrosis (IPF) and other fibrotic diseases, when administered using clinically feasible dosing regimens.
AdAlta CEO and Managing Director, Dr Tim Oldham commented:
“This new data is critically important. We have known AD-214 is efficacious in animal models of fibrotic diseases and also that it can at least partially block its target receptor forseveral days and even weeks after a single IV infusion. What we have not known is whether we could replicate the therapeutic effect seen in animals at these levels of receptor occupancy and hence at dosing frequencies acceptable in humans.”
“For the first time we have been able to show that we can maximally inhibit a key fibrotic process with as little as 60% receptor occupancy and that meaningful inhibition can be achieved at much lower levels. This supports the hypothesis that AD-214 may be able to be dosed at a clinically convenient frequency of no more than every two weeks. This information is also extremely valuable for determining appropriate dosing for AD-214 Phase II clinical studies.
“Linking a clinically measurable parameter (receptor occupancy) with efficacy answers a question commonly asked by potential commercial partners and substantially reduces the risk of Phase II studies. The completion of these difficult experiments is a credit to our inhouse scientific team.”
Study overview
AdAlta is developing AD-214 as a first-in-class treatment for fibrotic diseases such as IPF. AD-214 targets and blocks the chemokine receptor CXCR4 that is a receptor involved in several cellular processes involved in fibrosis, including the migration of immune, inflammatory and fibrotic cells to the sites of injury and disease.
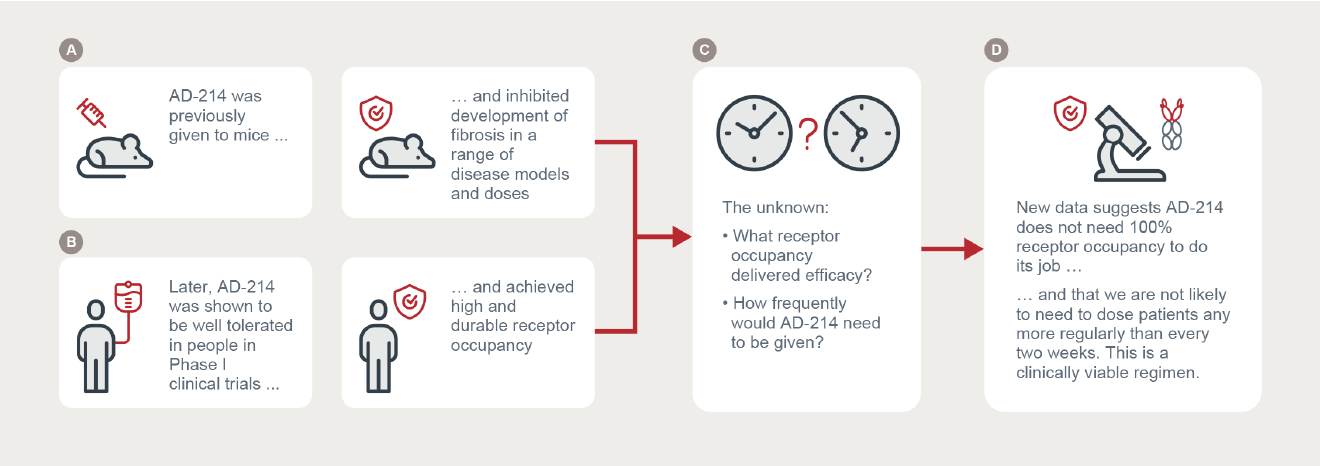
In earlier preclinical studies, AD-214’s efficacy was demonstrated in animal models of lung (see Figure 1 panel A), kidney and eye fibrosis, and AD-214 was found to inhibit several fibrotic processes. AD-214 was also shown to be safe and well tolerated in healthy volunteers in a Phase I clinical study. During that Phase I study, it was also observed that AD-214 achieved more than 50% blocking (or receptor occupancy) for at least a week at 5 and 10 mg/kg and up to three weeks at 20 mg/kg (see Figure 1 panel B). Clinical convenience requires at least two weeks between IV infusions. The optimal level of sustained receptor occupancy required for therapeutic effect was yet to be determined (see Figure 1 panel C).
The new ex vivo studies simultaneously measured AD-214’s ability to occupy CXCR4 receptors and to inhibit a key fibrotic process, the migration of cells expressing CXCR4 on their surface. AD-214 concentrations included those low concentrations observed in blood several days after IV infusion in Phase I clinical studies. The studies were able to replicate the CXCR4 receptor occupancy observed in Phase I clinical studies at these low concentrations. Significantly, even when receptor occupancy was substantially less than 100%, material inhibition of cell migration was observed.
The new data is the first time a clinically measurable parameter (receptor occupancy) has been linked to an antifibrotic mechanism (cell migration) and supports the hypothesis that intravenous administration of AD-214 every two weeks or more could be efficacious (see Figure 1 panel D). The results of the upcoming Phase I extension study and detailed pharmacokinetic simulation studies will now be able to optimise dosage and dose scheduling for Phase II studies with increased confidence, substantially reducing the risks associated with conducting those Phase II studies.
Figure 1: New AD-214 data links Phase I results with efficacy, informs dosing
Detailed study results
CXCR4 is a receptor involved in several cellular processes involved in fibrosis, including the migration of immune, inflammatory and fibrotic cells to the sites of injury and disease. Blocking CXCR4 is known to inhibit this migration. T cells, a type of immune cell that expresses CXCR4, are readily accessible from patient blood and serve as a model system for these processes.
In the recently completed studies, AdAlta’s scientists used human T cells from three different donors to study the link between AD-214 concentration, CXCR4 receptor occupancy and the ability of the CXCR4 expressing cells to migrate under normal signalling cues.
The ex vivo studies showed that:
- CXCR4 receptor occupancy measured in the model system at different AD-214 concentrations was consistent with the receptor occupancy observed in Phase I clinical studies at the same circulating blood concentrations of AD-214.
- The migration of human T cells in response to normal signalling cues can be inhibited by treating with AD-214.
- Maximal migration inhibition was achieved at CXCR4 receptor occupancy of 57-85% while a meaningful 50% migration inhibition was achieved at CXCR4 receptor occupancy of 11-37%.
- Maximal migration inhibition (and corresponding required receptor occupancy) was achieved at AD-214 concentrations of 0.05-0.07 μg/mL with 50% migration inhibition achieved at AD-214 concentrations five- to ten-fold lower.
- Blood concentrations of AD-214 exceed 0.07 μg/mL for approximately 72 hours following a single 10 mg/kg intravenous administration of AD-214, well after primary distribution from the blood.
Taken together, these results help identify target levels of CXCR4 receptor occupancy, and hence circulating concentrations of AD-214, that may be needed for efficacy in fibrotic indications. Maximal T cell migration inhibition, and hence potential efficacy against fibrosis, might be achieved by maintaining CXCR4 receptor occupancy above 60-85% and meaningful inhibition might be achieved as low as 11-37%. Due to the very tight binding of AD-214 to CXCR4, these levels of receptor occupancy can be achieved at very low circulating concentrations of AD-214. The Phase I clinical study shows that concentrations for maximal inhibition of T cell migration are maintained for several days after intravenous administration, and for meaningful inhibition for much longer.
This in turn supports the hypothesis that intravenous administration of AD-214 at clinically convenient two weekly dosing intervals could be efficacious.



