Posted 29 October 2020
This is a BioMelbourne Network Good News Story – click here to find out more and submit yours.
What do you do when you’re preparing to launch your surgical device into the US market and a global pandemic hits cancelling surgeries and impacting your market?
Well if you’re Livac Pty Ltd you leverage your existing position and keep going.
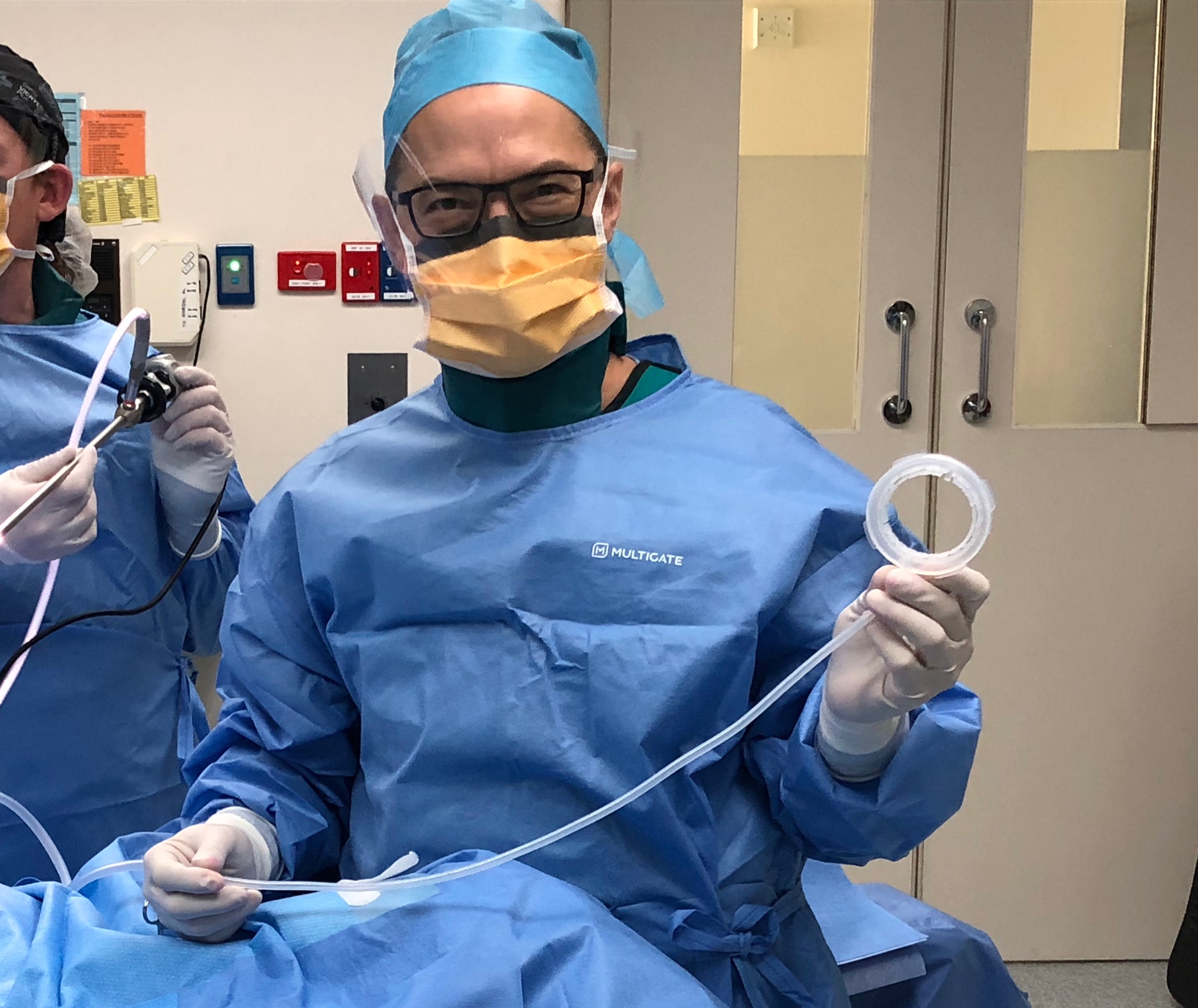
The Melbourne-based surgical device company has developed and commercialised a novel device, the LiVacTM Retractor, for use in upper abdominal surgery where the liver is required to be retracted. More than 12 million surgical procedures globally each year require liver retraction to enable the surgeon to access the underlying surgical field, presenting a > AUD 2.4 Billion market opportunity.
Whilst devices for organ retraction during surgery are not new, the LiVacTM Retractor is made from soft medical-grade silicone and utilises suction to provide gentle organ retraction, minimising trauma and recovery times for patients, ultimately leading to less post-operative pain medication and a shorter length of stay. The idea for the device coming from Victorian surgeon and LiVacTM inventor, Dr Philip Gan, as he witnessed the liver naturally ‘sticking’ to the diaphragm temporarily due to surface tension.
Having successfully developed and implemented a commercial path to market in Australia and selected European markets, the company reached a significant milestone when in December 2019 it achieved FDA clearance for the LiVacTM Retractor.
But as they embarked on executing their US market entry strategy the pandemic hit, cancelling non-essential surgery across the country. Overseas travel bans also made engaging with distributors and surgeons more difficult.
“It was a critical time for the company. We knew that we had to be agile and adapt to what was happening but at the same time still find and hit the right targets,” said Livac CEO, Dr Anabela Correia.
“To do this we leveraged our existing networks and relationships. We had been fortunate to be selected for the BioMelbourne Network’s Go-To-Market in the USA program and travelled to Medical Alley in 2019. In the US Livac met with key payers, providers and decision makers that helped form our US market entry strategy. The networking opportunities connected us to relevant contacts specifically in the surgical market. These as well as our existing connections and relationships have been invaluable in supporting our US market development activities, albeit from a distance.”
Leveraging these existing relationships, Livac appointed its first US distributor in May this year and the relationships formed through Medical Alley helped to establish Livac’s supply and logistics to this market.
The company was also fortunate to have selected a state in the US that was less impacted by the pandemic and maintained a commitment to keep hospitals open by prioritising high profit surgeries – which included their target market – bariatric surgery.
The LiVacTM Retractor has now been successfully evaluated in US patients with a leading bariatric surgeon with other key surgeons expressing interest.
Typically, Livac will travel to meet with its distributors prior to appointment and will invest heavily in training distributor sales teams and meeting with surgeons and hospitals. During COVID, these meetings and training initiatives have been conducted remotely with Livac’s Australian-based staff available during US hours to support the process.
“With our network and distribution partner in place, we’re confident we’ll be able to take the next steps to gain further surgeon support and hospital commercial approvals in the USA.” Dr Correia added.
For more information, please go to livac.com.au
Livac Pty Ltd would like to acknowledge the support of BioMelbourne Network, LaunchVic and Medical Alley for their Go-to-Market in the USA program.
Find out more about BioMelbourne Network’s 2019 Go-To-Market in the USA program.
Want to submit your own story? Find out more.