Updated: 25 November 2022

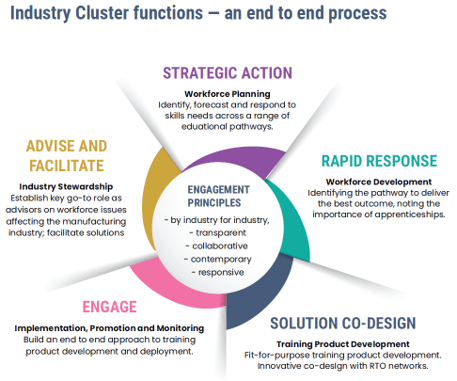
Be the go-to source for the healthtech industry and Victorian Government for data and insights on sector potential, priorities and needs
Work with the healthtech industry to identify and prioritise strategic projects and working groups and advisory panels that support industry transformation and strengthening of the local innovation ecosystem
Be the peak body for the Victorian healthtech industry to build and maintain a supportive policy, regulatory and investment environment
Often, the first three elements of our strategy strongly interrelate:
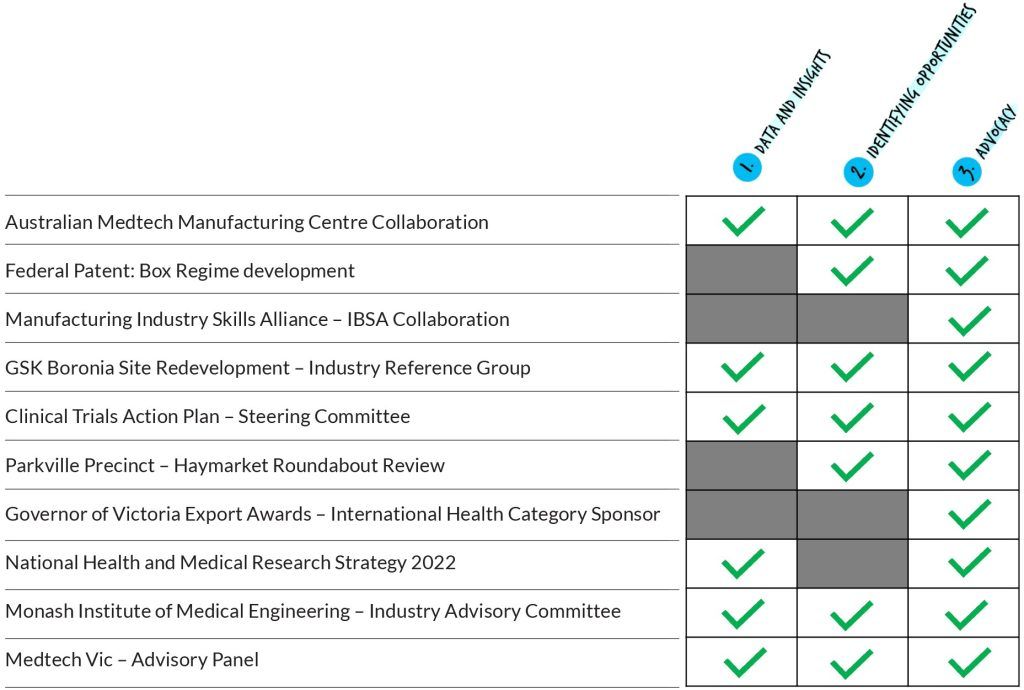
Australian MedTech Manufacturing Centre collaboration
BioMelbourne Network has developed a strong collaboration with the Victorian Government’s Australian Medtech Manufacturing Centre (AMMC).
In the first half of the 2022 calendar year, the new AMMC team is working to deliver on its three strategic priorities:
- grow local content into health procurement
- build the competitive capability of Victorian medtech manufacturers
- strengthen connections and collaboration across the sector.
Early in the year, BioMelbourne Network partnered with Dandolo Partners to map the Victorian Metech Product development infrastructure on behalf of AMMC. This was an exciting opportunity to map the entire medtech product development ecosystem in Victoria from the earliest stages through to final manufacturing and commercialisation.
This exercise resulted in an analysis of the sector’s strengths, weaknesses and opportunities. An overview of this report was presented at our BioSymposium: Optimising the Victorian Medtech Ecosystem on 5 April 2022. If you wish to view the event, please email info@biomelbourne.org.
Medtech manufacturers have identified multiple opportunities to improve capacity and collaboration in the sector, and to better connect manufacturers with medtech innovators. Through AMMC Partnerships funding, BioMelbourne Network will develop and run the first Healthtech Industry Community platform – called Wilam – to facilitate collaboration and connection across the life sciences industry in Australia.
Wilam will focus on manufacturing topics of interest and bridge the gap between manufacturers and innovators. This initiative is a direct response from industry leaders requesting more contemporary approaches to collaborate and engage with peers. The new community platform will connect companies, researchers, supply chain partners and service providers, creating new collaboration and project opportunities and amplifying existing initiatives.
AMMC is progressing work to leverage a key role for purchasers of medtech products to bring forward local solutions to address supply chain vulnerabilities. AMMC is partnering with the Industry Capability Network Victoria (ICN), which has appointed an ICN Medtech Adviser to work with Victorian medtech companies to identify new opportunities in local health procurement projects.
In 2022, AMMC has implemented two grant programs for the medtech sector:
- Medtech Manufacturing Capability Program for SMEs to expand or accelerate medtech manufacturing projects in Victoria
- Health-led Manufacturing Innovation Program to enhance collaboration between clinicians, manufacturers, medtech innovators, health services and researchers.
AMMC is also collaborating with partners to test four pilot projects and create new models of clinically led, demand-side innovation.
For more information about AMMC, visit the AMMC website.
For more information about the Wilam Life Sciences Community platform, visit the Wilam website.
Federal Patent Box Regime development
We believe that Australia’s policies on research investment and commercialisation need reforming so we can compete with the UK, Switzerland, France, Ireland and Singapore, which offer targeted incentives to encourage commercialisation of home-grown intellectual property. The introduction of a patent box in Australia would complement the existing R&D tax incentive by focusing on the revenue-generating phase of the medical research value chain.
In 2021, we collaborated with a national group called the Australian Medtech Manufacturing Consortium. This group comprises national and state-based industry associations, as well as major companies such as Cochlear, Resmed, CSL and others.
In August 2021, we completed our patent box regime submission to Treasury, ensuring that our messaging was aligned with other state and national industry bodies, including AusBiotech. We also helped prepare a joint statement of support for the Patent Box regime with Research Australia, AusBiotech and Medicines Australia, which was presented to Australian Government ministers and opposition ministers.
In May 2022, the Morrison Government had committed to the introduction of a patent box for Australian medtech and biotech companies. Unfortunately, this legislation was not passed by Parliament before the federal election.
BioMelbourne Network will continue to advocate for the introduction of this policy with the new Albanese Government.
Manufacturing Industry Skills Alliance – IBSA collaboration
The Commonwealth, States and Territories are working together to ensure a strong national training system that equips individual Australians and the Australian workforce with the skills needed for current, emerging and future jobs, opportunities and challenges.
Industry clusters will operate within the national training system and be accountable for high-quality training programs that address the skills needs of both employers and learners.
They will bring together industry leaders to look forward, drive performance and improve outcomes across and beyond the training system.
Industry owned/ Industry led
Manufacturing has a new sense of optimism and addressing skills issues is critical to the industry’s future in Australia.
IBSA Group have established a new entity, called the Manufacturing, Print and Textiles Industry Cluster, which will allow industry to play a strong role in workforce skills development.
This new alliance will engage and collaborate with employers, industry associations, unions and training organisations.
These life sciences industries will participate in discussions:
- pharmaceutical manufacturing
- advanced manufacturing and production
- basic chemical and chemical product manufacturing.
Why does this matter?
The manufacturing industry has told us we need to:
- Grow sovereign capability in areas of competitive advantage, driving a skilled workforce in new technologies, product development, design and prototyping, along with sustainability and collaborative skills.
- Elevate critical strategies to ensure labour mobility and quality. This will be possible if industry sectors partner together on an integrated manufacturing growth plan.
- Focus on new and emerging industries, prepare for future job roles and develop relevant training programs.
GSK Boronia site redevelopment – Industry Reference Group (IRG)
In June 2022, the Victorian Government Department of Jobs, Precincts and Regions (the department) invited BioMelbourne Network to join an Industry Reference Group (IRG), discussing potential options for the GSK Boronia site redevelopment, in Melbourne. We accepted this offer.
GSK will cease production at the end of 2022 with decommissioning activities planned in the second quarter 2023. The department in partnership with Charter Hall has engaged EY to develop an options opportunity piece to support the continuation of a Life Sciences Precinct at the Boronia site.
The IRG consists of participants from the Department of Jobs, Precincts and Regions, GSK, Monash University, MTP Connect, Knox City Council, Australian MedTech Manufacturing Centre and other industry leaders.
The group has been formed to:
- act as a governance mechanism and provide feedback about business cases and options for consideration by Charter Hall and the Victorian Government
- provide input about Life Sciences Precinct redevelopment options that are consistent with the Victorian Government’s Medtech and Pharmaceutical Sector Strategy to capture high-value manufacturing and build capabilities in medicine manufacture, rapid diagnostics testing, cell therapies, clinical trials infrastructure and vaccines
- provide insight and advice on the recommendations and options being developed by EY for consideration by Charter Hall and the Victorian Government
- provide strategic advice about retaining and expanding equipment and assets on the site, to increase the competitiveness of Victorian medtech manufacturers.
IRG meetings were held at GSK Boronia in June, and in Melbourne in July. At the second meeting, IRG workshopped a Vision Statement and heard the views of industry participants who provided case studies of life sciences precincts overseas.
The IRG will actively pursue development of opportunities from industry who wish to utilise the location for pharmaceutical or medical device manufacturing, research and development, education or other purposes.
If you are interested to learn more about the site or have an opportunity you’d like to discuss, please email BioMelbourne Network CEO Jeff Malone for an initial discussion or email Nick Lidonnici, Charter Hall Regional Portfolio Manager- Industrial and logistics.
Clinical Trials Action Plan – Steering Committee
As part of the Health and Medical Research Strategy (2022-32), the Clinical Trials Action Plan (CTAP) has been established to lead reform for clinical trials in Victoria. The CTAP will develop a forward-thinking roadmap of priorities which will implement commitments from the Health and Medical Research Strategy. This will help secure Victoria’s position as a world leader in clinical research.
The CTAP Steering Committee has been established as an advisory group to the Clinical Trials Action Plan project, which is managed by the Office of Health and Medical Research and Department of Jobs Precincts and Regions. It comprises leaders and stakeholders involved in Victorian clinical trials, including government, rural and regional healthcare, researchers and clinicians, biotech and pharmaceutical companies, patient engagement and Indigenous health.
As an organisation supporting the research community, BioMelbourne Network is delighted to be a participating member of the CTAP Steering Committee.
The CTAP Steering Committee will provide oversight of the CTAP project, including advice on project direction. They will provide expert review and feedback of project materials, highlighting relevant organisational activities and/or interdependencies, and identify relevant areas of consideration.
Here are some areas of reform that the CTAP Steering Committee will discuss:
- increasing the number of clinical trials in Victoria, including investigator-initiated trials and commercially-sponsored clinical trials
- improving clinical trial start-up times
- building capacity for rural and regional clinical trials
- increasing community engagement and patient recruitment to clinical trials, including Indigenous and minority communities
- building and supporting the clinical trials workforce.
Advice from the Steering Committee will contribute to clinical trials reform that will improve health and economic outcomes here, and secure Victoria’s position as a world-leader in clinical research.
Parkville Precinct – Haymarket roundabout review
BioMelbourne Network joined with 17 institutions resident in the Parkville Biomedical Precinct to advocate for the redevelopment and reform of the infamous Haymarket Roundabout on Flemington Road.
The Parkville Biomedical Precinct is now a globally recognised innovation precinct and home to medical research institutes, the University of Melbourne, University High School and four major hospitals. It will also soon be the new home for CSL, a biotech incubator, Australian Institute for Infectious Diseases, and the Parkville metro station.
All the new development biomedical plus the existing institutions are bringing rapid growth – generating jobs, economic development and research outcomes. But they are also exponentially significantly increasing the daily pedestrian, cyclist, motorist and public transport movements into and across the Haymarket Roundabout.
BioMelbourne Network has added its voice to a lobby representing the thousands of patients, clinicians, researchers, employees, and visitors who interact daily with the roundabout – and we are seeking an urgent review of the design of the roundabout, to enhance pedestrian and cyclist safety as well as facilitate improved thoroughfare, allowing the free flow of movement among precinct tenants.
Over and above that though, while the Parkville Biomedical Precinct is already world-class, we believe there is a real transformational opportunity to be had in the renovation and renewal of the roundabout. Geographical clusters of research, clinical, educational and industrial facilities – where the free flow of people is enabled and encouraged – actively drive and support the exchange of ideas and create a powerful cross-functional knowledge ecosystem. Further, whilst already the gateway to Melbourne, we believe the opportunity exists to make the roundabout into an iconic centrepiece of the precinct – safe, globally recognisable and synonymous with Australian health and medical research excellence.
For more information, read the letter from the Haymarket roundabout stakeholder group.
Governor of Victoria Export Awards – International Health Category sponsor
BioMelbourne Network is a proud sponsor of the Governor of Victoria Export Award in the International Health Category.
The annual Governor of Victoria Export Awards (GOVEA) recognise and celebrate excellence and success of Victoria’s best exporters. Open to all Victorian exporting businesses, GOVEA recognises and rewards leadership and innovation in international trade.
Now in its 43rd year, the prestigious GOVEA awards will boost a business’ reputation domestically and internationally and demonstrate to their clients that their business is recognised as a leading exporter in the state.
The International Health award category recognises outstanding international success in medical, healthcare, biotechnology fields for products, technology, equipment or services. This includes eHealth, digital health, medtech and aged care services.
BioMelbourne Network CEO Jeff Malone presented the 2022 GOVEA International Health award to SDI Limited to CEO Samantha Cheetham who also won the 2022 GOVEA Exporter of the Year award.
Congratulations.
SDI Limited is a Victorian manufacturer and world leader in research, development and manufacture of specialist dental materials that are sold to dentists in over 100 countries globally. SDI have achieved 90% of all sales to international markets, which is a terrific achievement.
National Health and Medical Research Strategy 2022
Greg Hunt, the former Health and Aged Care Minister announced his commitment to developing a National Health and Medical Research Strategy in 2022 during his keynote address at last year’s Research Australia Awards.
Research Australia has been working on the development of the strategy for several years.
After 20 reviews of health and medical research over the past 15 years, it’s time to develop a national strategy that will implement many of the recommendations that aim to sustain our sector, advance our capacity for innovation and meet future health challenges and healthcare needs.
Research Australia will work with key industry partners around the country to gain their perspective and to ensure that the outcome is sensible for us all.
BioMelbourne Network plays a vital role in our ecosystem and any reforms will need to reflect our constituency as well. We were involved in the review process with Research Australia in the first quarter of 2022 prior to the report’s publication before this year’s Federal Election.
For more information, read Research Australia’s statement on behalf of Australia’s health and medical research sector.
Global Talent Visa
Access to skilled talent within the sector remains a significant challenge.
Last year, BioMelbourne Network participated in discussions with the Federal Global Business and Talent Attraction Taskforce
about the availability of their Global Talent Visa and Post COVID-19 Economic Recovery Visa programs, with the Prime Minister’s Special Envoy for Global Business and Talent Attraction, the Australian Government, along with Marilyn Jones, from Mexec, The Migration Agency Managing Director Sarah Thapa, and other state and national industry associations.
We can confirm that the program is open through this year and talented individuals in the health and life sciences field can apply with nomination from an Australian organisation or industry body.
Some important facts:
- One of the key benefits of this program is that once a Global Talent Visa is issued individual and direct family members immediately have permanent residence status.
- There are 8,448 places allocated for 2022-23. Last year, there were 15,000 visa places available, which were possibly underutilised, resulting in a lower limit this financial year.
- The primary focus of the Global Talent Attraction Taskforce this year is to connect organisations with Global Talent whereas previously, the Taskforce also focused on attracting individuals without employment connections in Australia.
- 60% of visas were issued to people working in digital technologies and health/ life sciences sectors.
- The Taskforce will also offer Post COVID-19 Economic Recovery Visas (subclass 408), also known as beach head visas, to key staff working for companies that are setting up a subsidiary in Australia, allowing for an 18-month window.
Talented individuals in the health and life sciences field can apply with a nomination from an Australian organisation or industry body.
For more information about visa eligibility, visit Global Australia.
We have also recorded a podcast about this national Global Talent Visa program and a similar program in Victoria.
For more information about these important programs, listen to our podcast on Special Visa Schemes.